This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Final Advice Clinical studies with questionnaires
Clinical studies with questionnaires (outside SoC): Interventional vs non-interventional?
From BAREC, we give an advice on how to consider studies involving questionnaires (outside standard of care (SoC)) and which fall under the (Belgian) law of 7 May 2004 to be interventional or non-interventional.
No additional diagnostic or monitoring procedures will be proposed in these studies. Participants will only be requested to complete a questionnaire, and potentially, data can be collected from their medical records.
BAREC wants to highlight that questionnaire completion should not result in longer duration of consultation room occupancy, and should be done at a pre-specified, convenient location with the necessary comfort and privacy for the participant.
The below advice is not applicable to anonymous questionnaires and it is not applicable to quality improvement projects. It’s applicable to studies in which the questions are processed pseudonymously and which fall under the (Belgian) law of 7 May 2004.
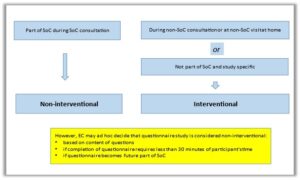
Please note that interventional studies with questionnaires that also investigate the safety or efficacy of one or more medicinal products, which all have a marketing authorization, are covered by the Clinical Trial Regulation (CTR) and therefore not by the (Belgian) law of 7 May 2004. We refer to the definition of a low-intervention clinical trial according to article 2 (2)(3) of the CTR.
